Physico-chemical studies of the interaction of antibiotic drugs with surfactants
(a) Characterize the interactions of cefixime trihydrate drug with DTAB by various techniques
(b) Observe the effect of temperature, salts and solvent on the drug mediated surfactant micellization and clouding processes
(c) Thermodymanics of micellization processes
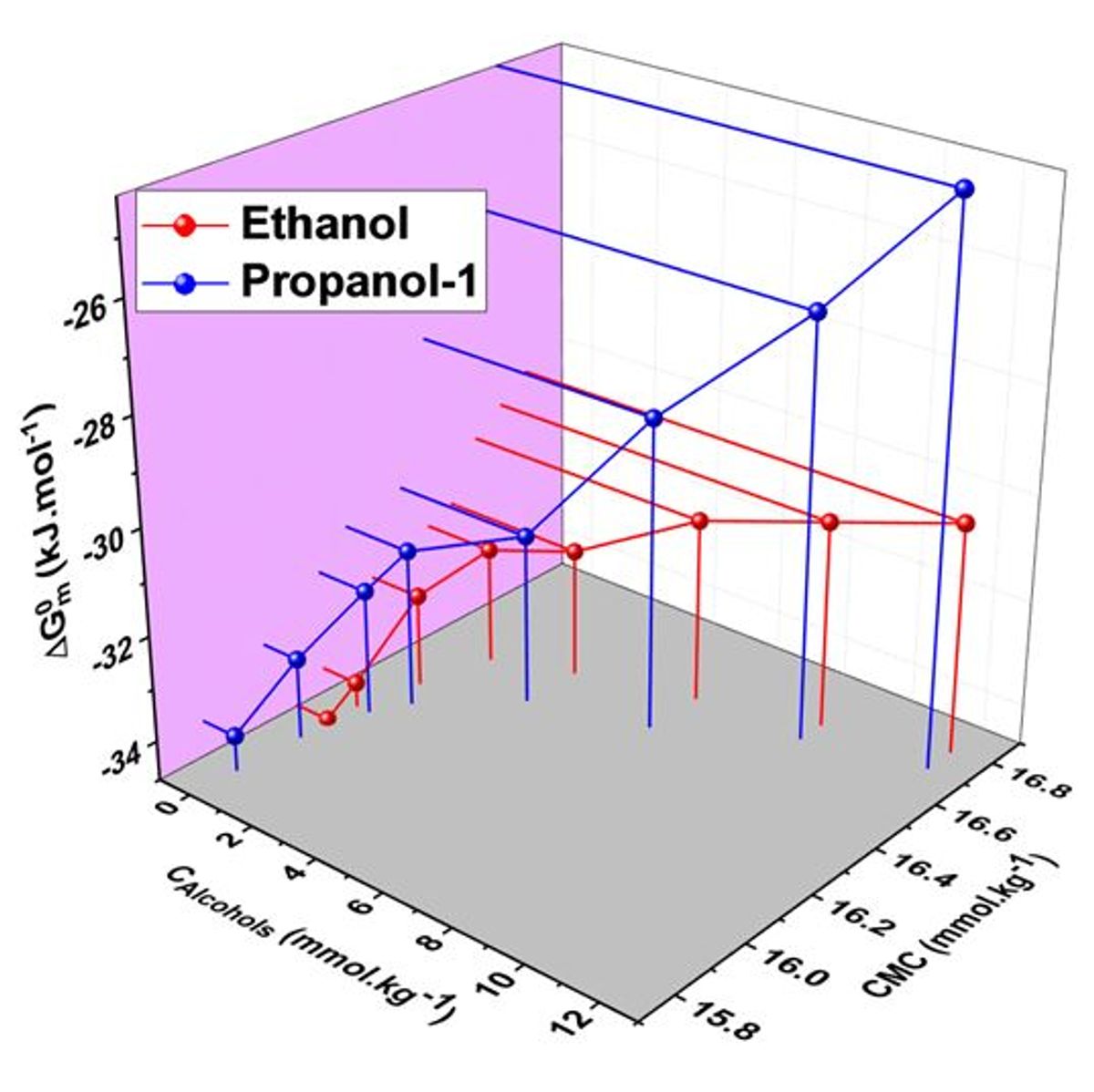
The project aims at determining the micellar parameters such as such as critical micelle concentration (cmc), ideal value of the critical micelle concentration (cmcid), micellar mole fractions (X1Rub) and their ideal values ( ), activity coefficients ( and ), degree of dissociation (g) as well as different thermodynamic parameters such as (standard free energy change (ΔGmo), standard enthalpy change (ΔH0m), standard entropy change (ΔS0m) of micellization as well as excess free energy of micellization ( )) in pure water as well as in aqueous solution of different additives such as alcohol, salt, etc.

